EU / UK COSMETICS RESPONSIBLE PERSON SERVICE
Get your product launch ready in 5 days with no headaches.
We guide cosmetic brands through EU/UK compliance — from safety assessments and responsible person services to notifications and documentation.

COSMETICS COMPLIANCE, ALL INCLUSIVE SERVICE
Your single cosmetic consultant for worldwide market access
We support cosmetics brands at every stage — from first market entry to worldwide multi-country expansion. In one package you get a launch-ready, hassle free setup without managing multiple providers or hidden costs.
PRICE OVERVIEW
Responsible Person Service
$270 per year*
* Example price for one product. For more than 3 products, we offer highly discounted rates.
You will receive:
-
ONE OFF per product $150 Product Information File (PIF) + Cosmetic Product Safety Report (CPSR)
- Authoring file from scratch
- Includes ingredient + label review
- Includes both Part A - CPSI and Part B - CPSA -
FREE EU CPNP notificationas your responsible personOtherwise $40 per CPNP
-
FREE UK OPSS/SCPN product notification
as your responsible person
Otherwise $40 per OPSS/SCPN -
FREE Product label & packaging compliance review
as your responsible person
Otherwise $150 / product -
Launch-ready within 5 business days
.webp?width=110&height=110&name=free_cosmetics_formula_no_background_optimized%20(1).webp)
1. Free Formula Assessment

2. Formula accepted EU/UK

3. Documents check

4. Getting
started
.webp?width=110&height=110&name=product_label_no_background_optimized%20(1).webp)
5. Packaging & Label review

6. PIF & CPSR
creation

7. CPNP Notification
WHY OUR APPROACH MAKES MORE SENSE?
OUR APPROACH
Before you pay: We will guide you on the correct path - no strings attached.
-
Recieve our compliance roadmap tailored to your products, before you commit to our service.
-
Talk to compliance experts with ecommerce expertise.
-
Keep it simple - only pay for what is minimally needed.
-
Marko our compliance manager is already working on your case, just submit your inquiry now.
OTHERS
No clear path upfront - headaches and long waiting time
-
Talk to sales not to compliance.
-
Pay upfront, be told later you can not sell your product in this market.
-
No peace of mind.
-
Your case number is #328219 ;) Our sales department will route your request and get back to you in several business days.
ALL YOUR QUESTIONS ANSWERED BEFORE YOU COMMIT
THE NEW WAY OF COMPLIANCE
%20(1).webp?width=400&height=279&name=happy_man_no_background_optimized%20(CUT)%20(1).webp)
.webp?width=400&height=276&name=stressed_man_background_optimized%20CUT%20(1).webp)
| EU Compliance Partner | Big Law & Traditional Firms |
|---|---|
| ✔️ We understand the unique problems of ecommerce sellers. The law and the practical real world ways of doing business are 2 completely different things. | ❌ We are lawyers and have no real world ecommerce understanding. |
| ✔️ We pick up the phone | ❌ 7–10 days to hear back (if at all). You’re just another case number |
| ✔️ Built for speed & ecommerce reality - Fast decisions, fast execution | ❌ Built for paperwork, not sellers - Slow processes, internal approvals |
| ✔️ Personalized service for every client | ❌ Indirect, standardized handling |
| ✔️ We understand Amazon Seller Central, Shopify & other marketplaces and will guide you with practical experience | ❌ No real marketplace experience |
WHAT WILL YOU GET?
A compliant cosmetics label and packaging. A CPNP registration number. Including full cosmetics documentation. A reliable partner and calm mind for future growth.
.png?width=600&name=COSMETICS%20LABEL%20(1).png)

⚠️WHAT HAPPENS IF YOU DON'T COMPLY⚠️
You risk full liability in case of consumer issues, custom inspections or delays. Depending on the country, fines can range from €10.000 to €100.000 or even higher considering product recalls, import bans and criminal liability. In the EU and UK, cosmetics are strictly regulated. If your product is intended to clean, protect, perfume, change appearance or care for skin, hair, or nails it must comply with cosmetics law — otherwise, it cannot be legally sold.
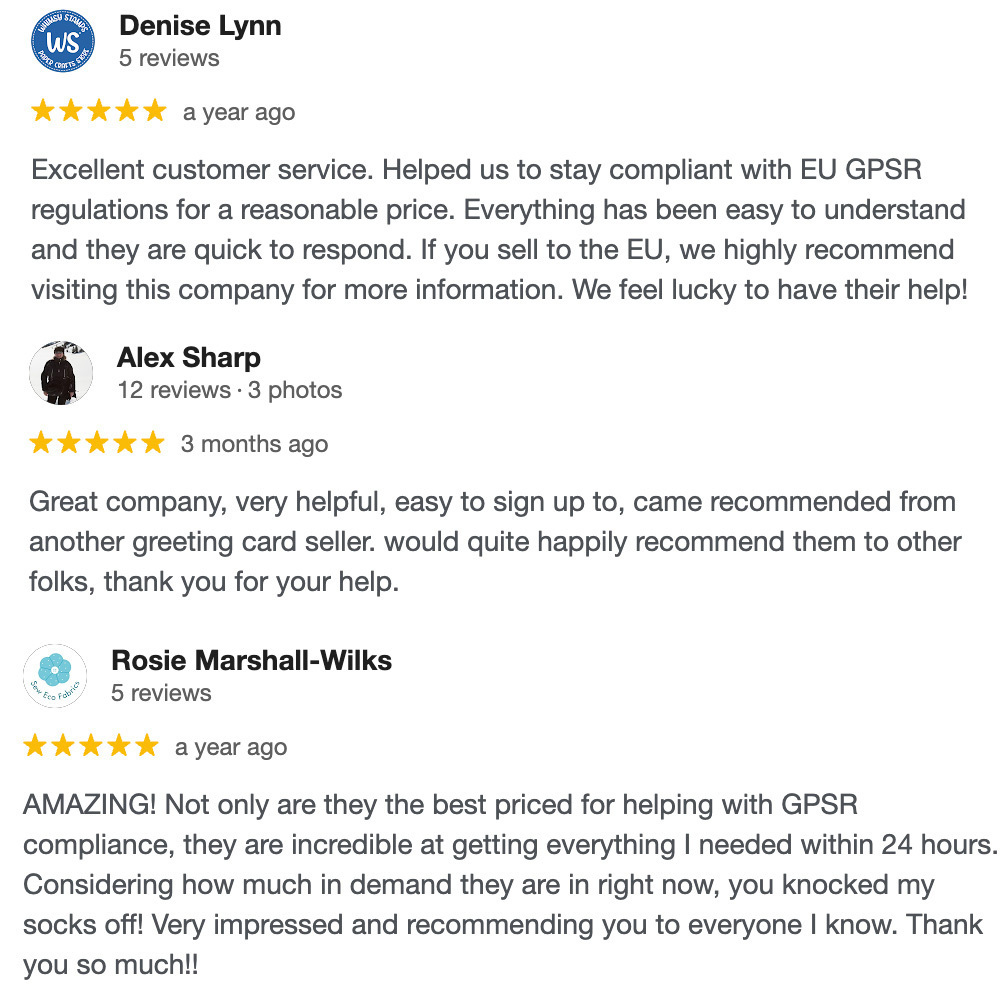
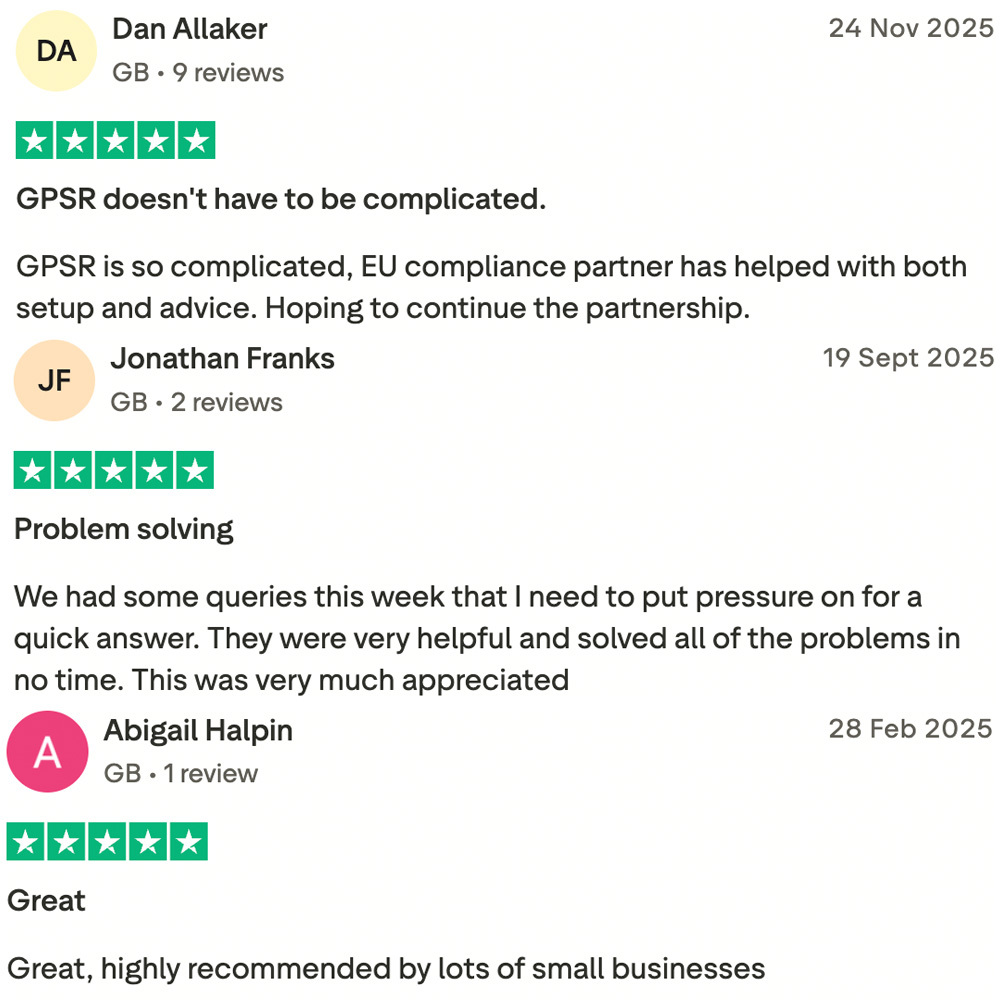
WHAT OUR CUSTOMERS SAY:
READY TO START SELLING?
Fill out the Form, we get started right away, review your reuqest and get back within 48 hours.
FAQ
What is the best way to send product information over?
The best and fastest way is a structured "digital package", ideally shared via Google Drive, Dropbox, or similar.
We recommend one clearly named folder per product(group), containing:
-
Final label artwork
-
Ingredient list (INCI format)
-
Product formula with exact percentages
-
Safety documents (SDS, COA, IFRA if fragrance is used)
-
Product description and intended use
-
Manufacturer details
Authorities and safety assessors work document-by-document, not from emails or screenshots. A structured folder avoids delays, misinterpretation, and re-requests.
What documents are required for cosmetic products?
For EU and UK cosmetics, the minimum required documents are:
-
Cosmetic Product Safety Report (CPSR)
-
Part A: Safety information
-
Part B: Safety assessment (signed by a qualified safety assessor)
-
-
Product Information File (PIF)
(explained in detail below) -
CPNP (EU) or SCPN (UK) notification
-
Online notification before placing the product on the market
-
-
Label artwork
-
Must meet all mandatory labeling requirements
-
-
Manufacturing information
-
GMP compliance (ISO 22716 or equivalent)
-
Without these, a cosmetic cannot legally be sold, even if it’s already on Amazon or Shopify.
Do we need different artwork for the UK and EU?
In most cases - YES.
Why:
-
The Responsible Person address must be local:
-
EU product → EU Responsible Person address
-
UK product → UK Responsible Person address
-
-
The notification systems are different:
-
EU → CPNP
-
UK → SCPN
-
What might stay the same:
-
Design
-
Ingredients
-
Claims
-
Warnings
What usually changes:
-
Responsible Person name and address
-
Occasionally language or regulatory phrasing
What is the PIF (Product Information File) and what is required?
The Product Information File (PIF) is the legal backbone of a cosmetic product.
Authorities can request it at any time, and it must be provided without delay.
A compliant PIF includes:
-
Product description
-
Cosmetic Product Safety Report (CPSR)
-
Manufacturing method and GMP statement
-
Proof of effect for any claims (e.g. “anti-hair loss”, “moisturising”)
-
Animal testing statement
-
Label artwork
-
Ingredient and raw material documentation
Important:
-
The PIF must be kept for 10 years
-
It must be available in the EU or UK, depending on the market
What specific warnings or statements are needed on the label?
Warnings depend on ingredients and product type, but the following are common:
Mandatory for all cosmetics:
-
Function of the product (unless obvious)
-
Ingredient list (INCI)
-
Responsible Person name & address
-
Nominal content
-
Batch / lot number
Conditional warnings:
-
“For external use only”
-
“Avoid contact with eyes”
-
“Keep out of reach of children”
-
Any Annex III restricted ingredient warnings (mandatory wording)
Key point:
Warnings are not optional or “nice to have” — if an ingredient requires one, it must appear exactly as prescribed.
Is the date of minimum durability or period after opening required on the label?
Yes – one of them is mandatory.
Rules:
-
If shelf life is less than 30 months →
Date of Minimum Durability (“Best before” date) -
If shelf life is more than 30 months →
Period After Opening (PAO) symbol (e.g. 12M, 24M)
This is non-negotiable and frequently checked by authorities.
Is the recycling symbol required on the actual shampoo bottle or the master carton?
Short answer: It depends on the market and packaging type.
-
Cosmetics law itself does not mandate recycling symbols
-
Packaging waste laws (EPR) do
Common practice:
-
Recycling symbols are placed on consumer-facing packaging
-
If the bottle is sold alone → bottle needs marking
-
If bottle + outer box → usually both are marked
In many EU countries, missing recycling information can still trigger fines, even if the cosmetic itself is compliant.
Does the manufacturer need to be listed on the label?
Not necessarily.
What is mandatory:
-
Responsible Person name and address
Manufacturer details:
-
Can be inside the PIF
-
Do not need to appear on the consumer label unless used for marketing
Are translations required for the label?
Yes.
Rule:
-
Mandatory label information must be in the official language(s) of the country of sale
Examples:
-
Germany → German
-
France → French
-
Italy → Italian
What must be translated:
-
Function
-
Warnings
-
Instructions
-
Durability information
What does not need translation:
-
INCI ingredient list (always Latin/English)
Is saw palmetto considered a medicinal product or cosmetic?
Saw palmetto has specific implications when used in products and requires clarification regarding its classification, as it may have medicinal properties that affect product classification.



